
Testing Principle
When Lp(a) in human serum is exposed to specific monoclonal antibody, the corresponding antigen-antibody complexes can be formed, making for an increased turbidity of the sample. The turbidity is proportional to the concentration of Lp(a) in the presence of the monoclonal antibody. The concentration of Lp(a) in the samples can be determined by comparing to the standard sample in the specific wavelength.
Product Features
Linearity range: 30 – 1000 mg/L; linear correlation coefficient ≥0.990; linearity deviation is within ±10%.
Absorbance of blank ≤ 0.80
Sensitivity: the difference of absorbance per unit of concentration (ΔA) ≥ 0.03 while testing the specific sample of concentration of 100 mg/L.
Accuracy: Relative deviation is within ±10%.
Precision: intra assay CV(%) ≤ 5%;inter assay CV(%) ≤ 5%.
Technical Parameters
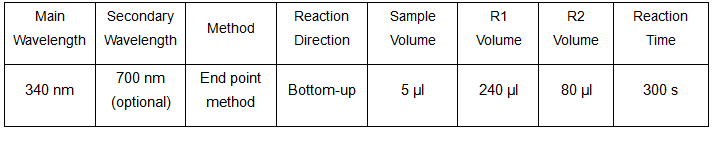
Reference range: 43 – 287 mg/L
Packaging Specification
R1: 1×30 ml, R2: 1×10 ml
R1: 2×30 ml, R2: 1×20 ml
R1: 2×45 ml, R2: 2×15 ml
R1: 2×60 ml, R2: 2×20 ml
R1: 4×60 ml, R2: 4×20 ml
R1: 3×80 ml, R2: 4×20 ml
Calibrator: appendant
Quality control: additional purchase
Storage and Period of Validity
Store the Biomedia Lp(a) test kits at 2 to 8 ℃ protected from light. The period of validity of unopened reagents can be up to 16 months. Opened reagents can be valid for 1 month if stored at 2 to 8 ℃ protected from light